Abstract
Introduction: The optimal initial management of mantle cell lymphoma (MCL) is unknown, but aggressive induction chemoimmunotherapy followed by autologous stem cell transplant confers long remission durations and is standard for younger patients. Cytarabine-based induction achieves higher rates of MRD-negativity compared to anthracycline-based induction regimens and correlates with improved outcomes. Whether a similar benefit exists after bendamustine-rituximab (RB) induction is unknown. S1106 compared induction R-HyperCVAD/MTX/ARAC (RH) or RB followed by autologous stem cell transplantation (ASCT). We previously reported similar 2-year (yr) progression-free survival (PFS) and overall survival (OS) with either regimen, and provocatively found that MRD negativity was similar with both regimens. RH was more toxic than RB and had higher stem cell mobilization failure rates.
Methods: Inclusion criteria were untreated stage III, IV or bulky stage II MCL, Cyclin D1 +, age > 18-65, and adequate organ function. Randomization was stratified by MIPI. Patients (pts) received either 4 cycles of RH or 6 cycles of RB followed by ASCT. MRD was assessed at baseline and post induction. Extraction of genomic tumour DNA from paraffin embedded tissue or bone marrow aspirate and PCR amplification of IGH-VDJ, IGH-DJ, and IGK regions was performed followed by high-throughput sequencing to determine the tumor clonotype(s) (Adaptive Biotechnologies). DNA from peripheral blood mononuclear cells (PBMC) and plasma was amplified and sequenced to determine lymphoma molecules per million diploid genomes. Herein we report the results of the updated analysis at a median follow-up (mFU) of 5 yrs.
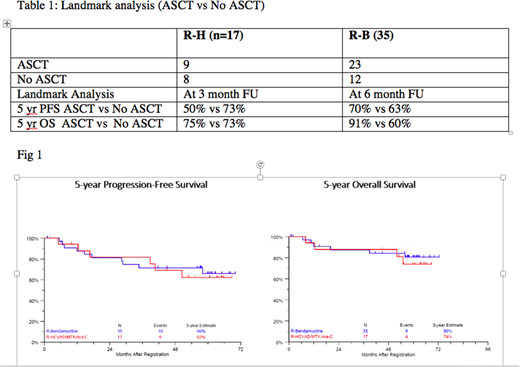
Results: Fifty-two of 53 pts were evaluable. Baseline characteristics were similar between the two groups except for more female pts in the RH group. This study was closed based on prespecified criteria of stem cell mobilization failures in the RH arm. With mFU of 5 yrs (range, 29 days-6yrs), updated ORR (94.1% RH vs. 85.7% RB) and CR (41% RH vs. 43% RB) rates were similar. The 5-yr estimated PFS was 62% and 66% and the 5-yr estimated OS was 74% and 80% for RH and BR, respectively (Fig.1). Increased grade 3/4 toxicity and inadequate stem cell mobilization was seen in the RH arm thus challenging its use as an induction platform for future upfront MCL trials. 9/17 pts in RH and 23/35 pts in RB arm underwent ASCT. A landmark analysis was performed evaluating 5-yr PFS and 5-yr OS with and without ASCT in each arm. (Table 1) 27 patients consented to optional MRD assessment, with 12 paired serial samples (baseline and post induction). Overall, the estimated 5 yr PFS was 90% for all patients who achieved MRD negative status at the end of RB. Long-term toxicity included acute myeloid leukemia (N=1) in the RH arm at 5.4 yrs after diagnosis and lung cancer (n=1) in the RB arm at 2.9 yrs after diagnosis.
Conclusion: The optimal induction regimen prior to ASCT in the initial management of MCL is a source of significant debate. Our initial findings supported RB as an effective platform with the ability to achieve MRD negativity. Long-term results of this study continue to demonstrate excellent response rates, 5-yr PFS and 5-yr OS with either RH or RB without any new toxicity signal. The 5-yr outcomes with RB compare favorably to more aggressive cytarabine-based induction regimens. Thus, R-Bendamustine could be an excellent backbone for induction therapy in transplant eligible patients and needs to be tested in larger phase III trials.
Support: NIH/NCI grants CA180888, CA180819, CA180821, CA180820, and in part by Sequenta, Inc. (Adaptive Biotechnologies)
Kamdar:Seattle Genetics: Speakers Bureau; Genentech: Consultancy. Chen:Millennium Pharmaceuticals: Consultancy, Research Funding; Affimed: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck & Co., Inc.: Consultancy, Research Funding, Speakers Bureau; Genentech Inc.: Consultancy. Rimsza:NanoString: Other: Inventor on the patent for the Lymph2Cx assay. Barr:AbbVie, Gilead: Consultancy. Phillips:Genentech: Consultancy; Seattle Genetics: Consultancy; Bayer: Consultancy; Gilead: Consultancy; Pharmacyclics: Consultancy, Research Funding; Abbvie: Research Funding. Leonard:ADC Therapeutics: Consultancy; Karyopharm: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy; Juno: Consultancy; Sutro: Consultancy; AstraZeneca: Consultancy; BMS: Consultancy; Novartis: Consultancy; Celgene: Consultancy; MEI Pharma: Consultancy; United Therapeutics: Consultancy; Biotest: Consultancy. Kahl:Seattle Genetics: Consultancy; AstraZeneca: Consultancy; Gilead: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Acerta: Consultancy; CTI: Consultancy; Juno: Consultancy; ADC Therapeutics: Consultancy; Genentech: Consultancy. Friedberg:Bayer: Honoraria. Smith:Portola: Honoraria; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal